IBM has revealed that graphene can't yet fully replace silicon inside CPUs, as a graphene transistor can't actually be completely switched off. ... Unlike silicon, 'graphene does not have an energy gap, and therefore, graphene cannot be “switched off," resulting in a small on/off ratio.
- Why is graphene not used in electronics?
- Why is graphene not used?
- Is graphene used in computers?
- Why would Graphene be a good substitute for silicon in microchips?
- Is Graphene the future?
- Can graphene stop a 50 cal?
- How much is 1g of graphene?
- How expensive is graphene?
- Who is the largest producer of graphene?
- Can graphene stop a bullet?
- What is the best graphene stock to own?
- Why is graphene better than silicon?
Why is graphene not used in electronics?
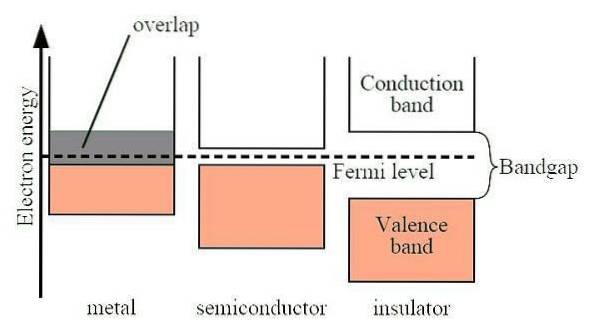
As previously mentioned, graphene is highly conductive - it can conduct electrons at nearly the speed of light, which is 100 times faster than any other known materials. However, for many applications in electronics, it is actually too conductive, as it has no band gap.
Why is graphene not used?
As /u/NanoChemist pointed out, there are problems in making "pristine" single layer graphene. But the main reason why it's not being used is that it's too new and technologies for processing and patterning it are still in relatively early stages of development.
Is graphene used in computers?
Essentially, graphene is a 2D sheet made of carbon atoms that has incredible properties of tensile strength and conductivity making it the thinnest yet the strongest martial. It conducts heat better than any other material and is also an excellent semi-conductor, making it a great material for computers.
Why would Graphene be a good substitute for silicon in microchips?
Microchips that use graphene can sustain many more transistors than commonly used materials like silicon. This alone will make electronics more efficient. The real benefit that graphene can provide is in the elimination of carbon created in silicon refinement.
Is Graphene the future?
Endless Possibilities With such astounding properties, graphene is predicted to be the material that changes the world. Scientists are hoping to develop stronger more powerful batteries that are so small they could be sewn into your clothes, or even your skin!
Can graphene stop a 50 cal?
Graphene is essentially one atom thick layers of graphite in a crystallinne formation. Lab tests have shown that just 4 one atom thick sheets can stop an AK-47 round. ... Graphene is expensive, and takes time to produce, but if you've got the budget, you could make a shield capable of blocking a 50. BMG.
How much is 1g of graphene?
Currently the cost of making one gram of graphene is somewhere around $USD100. But Australian scientists believe that they know a way to bring the cost way down to just 50 cents per gram.
How expensive is graphene?
There are processing stages that add to the cost beyond the $100 mentioned. However, as graphene currently ranges at anywhere between $67,000 and $200,000 a ton, there is a lot of potential to significantly reduce the cost of graphene products—perhaps, by even up to an order of magnitude.
Who is the largest producer of graphene?
Table 2: Global Manufacturers of Graphene
| Company Name | Location | Market Cap* |
|---|---|---|
| Saint Jean Carbon Inc. | Canada | $ 94.7 million |
| Haydale Graphene Industries | United Kingdom | $ 51.6 million |
| Group NanoXplore Inc. | Canada | $ 20.3 million |
| Graphene NanoChem LLC | United Kingdom | $ 7.1 million |
Can graphene stop a bullet?
Graphene Can Stop a Speeding Bullet. When fired, the bullets flew through the air at 600 meters per second, according to the researchers). Upon impact, the sheets of graphene absorbed twice as much impact as Kevlar, the material commonly used in bullet-proof vests, and did tens time better than steel.
What is the best graphene stock to own?
Top graphene stocks to watch
- Applied Graphene Materials.
- Haydale Graphene Industries.
- Versarien.
- Graphene NanoChem.
- G6 Materials.
Why is graphene better than silicon?
Graphene has many properties (in all forms) that make it an ideal material for electronic devices, ranging from its superior electrical conductivity properties to its high charge carrier mobility and its large and active surface area. Unlike silicon, graphene does not have a bandgap, making it highly conductive.
 Naneedigital
Naneedigital